
The scientific community has been galvanized by the announcement of the medicine nobel prize 2025, honoring three scientists whose work fundamentally redefined our understanding of the human immune system. Mary E. Brunkow, Fred Ramsdell (both USA), and Shimon Sakaguchi (Japan) were jointly awarded the prestigious prize for their “discoveries concerning peripheral immune tolerance.”
This groundbreaking research unveiled the crucial mechanisms that prevent our powerful immune defenses from mistakenly attacking the body’s own tissues—a condition known as autoimmunity. Their collective work identified the immune system’s ultimate “security guards”: Regulatory T-cells (Tregs), establishing a new pillar in the field of modern immunology.
The significance of this discovery extends far beyond basic research, offering profound new avenues for treating devastating diseases.
Key takeaways from the Nobel Assembly’s decision include:
- Identification of Regulatory T-cells (Tregs): These specialized cells actively suppress immune responses, ensuring self-tolerance in peripheral tissues.
- The Foxp3 Master Gene: The laureates linked a specific gene, Foxp3, to the development and function of these regulatory cells, explaining the root cause of certain autoimmune disorders.
- New Therapeutic Avenues: Their findings are directly inspiring treatments for autoimmune diseases, cancer immunotherapy, and successful organ transplantation.
The Problem of Self-Tolerance: Why the Immune System Needs a Brake
For decades, the prevailing scientific belief regarding how the body prevents autoimmunity—a process called “central tolerance”—was incomplete. Scientists knew that potentially harmful immune cells were eliminated during their development in the thymus gland. However, some self-reactive T-cells inevitably escape this weeding process and circulate throughout the body.
If these escaped “soldiers” were left unchecked, they would trigger widespread autoimmune destruction. The work that secured the nobel prize for medicine 2025 provided the critical second layer of defense, known as “peripheral immune tolerance.” This research proved that the body possesses an active, continuous mechanism to keep stray T-cells in check.
The Nobel Committee emphasized that without this regulatory system, the powerful immune system would inevitably attack our own organs, leading to catastrophic illness. The laureates’ work answered the fundamental question: How does the immune system differentiate between ‘friend’ and ‘foe’ outside the central control of the thymus?
Shimon Sakaguchi Nobel Prize Breakthrough: The T-cell Police
The journey to the medicine nobel prize 2025 began with the pioneering work of Shimon Sakaguchi in the mid-1990s. Sakaguchi challenged the established dogma by proposing that immune tolerance was not merely a passive result of cell deletion, but an active process carried out by a specific, dedicated cell type.
Through meticulous experiments, Sakaguchi isolated a previously unrecognized class of T-cells that carried the protein marker CD25 on their surface. He demonstrated that when these cells were transferred into immune-deficient mice, they actively prevented the development of autoimmune disease. He had discovered the “immune system’s security guards,” officially naming them Regulatory T-cells (Tregs) in 1995.
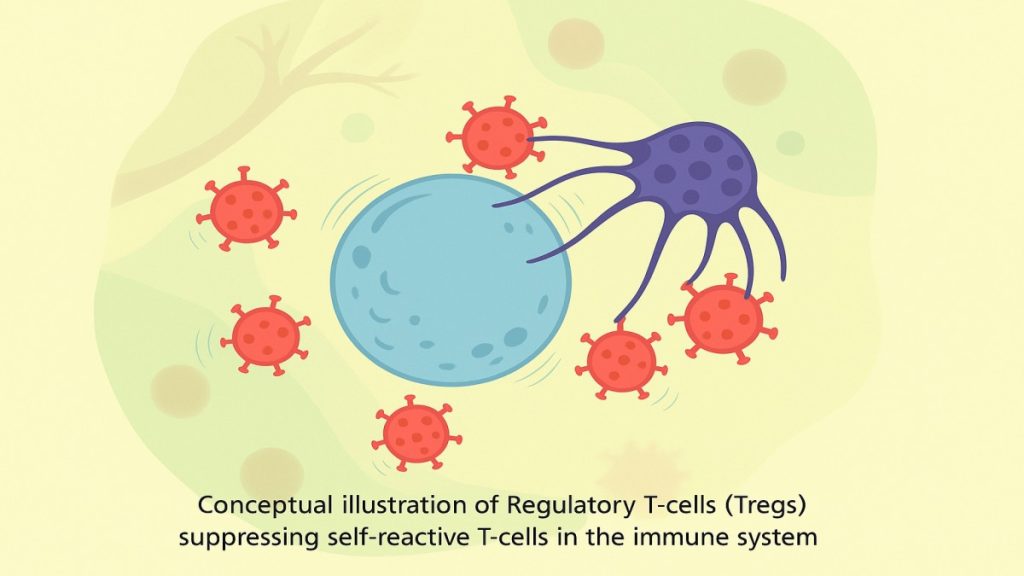
This discovery was initially met with skepticism in the global immunology community, as it overturned decades of accepted understanding. Yet, Sakaguchi’s evidence was robust, forcing researchers worldwide to reconsider the complexity of immune regulation.
The Foxp3 Gene: Unlocking the Regulatory Key
The final, essential pieces of the puzzle were provided by the American scientists, Mary E. Brunkow and Fred Ramsdell, in 2001. Working independently of Sakaguchi, they were studying a specific strain of mice known as “scurfy” that developed fatal, severe autoimmune diseases.
Brunkow and Ramsdell successfully pinpointed the genetic mutation responsible for this disorder: a defect in a gene they named Foxp3. They quickly established that mutations in the human equivalent of this gene caused the rare and devastating autoimmune disorder known as IPEX syndrome (Immunodysregulation, Polyendocrinopathy, Enteropathy, X-linked syndrome).
The critical connection was made soon after. Sakaguchi built on Brunkow and Ramsdell’s discovery, definitively showing in 2003 that the Foxp3 gene acts as the master regulator for the shimon sakaguchi nobel prize-winning T-cells. It controls the development and function of Tregs. This genetic link provided the irrefutable evidence that confirmed the central role of regulatory T-cells in maintaining peripheral immune tolerance.
From Discovery to Drug Development: The Therapeutic Promise
The combined discoveries of Brunkow, Ramsdell, and Sakaguchi have profoundly impacted biomedical research, opening two major therapeutic pathways:
1. Targeting Autoimmunity
Autoimmune diseases, such as Type 1 diabetes, rheumatoid arthritis, and multiple sclerosis, are characterized by insufficient immune tolerance, where the body’s defenses attack healthy cells. By understanding Tregs, scientists can now focus on strategies to boost their function.
- Increasing Treg Count: Research is underway to develop therapies that increase the number or enhance the activity of a patient’s own Tregs. This could involve cell therapies where Tregs are harvested, expanded in the lab, and re-introduced to stop the immune attack. As reported by major news organizations, these translational efforts represent the most exciting next phase of this basic science breakthrough (see the Reuters coverage on immune therapies).
2. Advancing Cancer Immunotherapy
In oncology, the goal is the opposite. It has been shown that tumors often co-opt Tregs, using them as a shield to protect themselves from killer T-cells that would otherwise destroy the cancer.
- Destroying Tregs in Tumors: For effective cancer treatment, researchers are designing drugs to specifically suppress or eliminate Tregs within the tumor microenvironment. Removing these “security guards” allows the immune system to launch a full and effective assault against the malignant cells. This area of research, often called immuno-oncology, has become one of the fastest-growing fields in medicine, following foundational work in immunotherapy (read more on this in the BBC Science section).

The work recognized by the nobel prize medicine is not just about historical science; it is the blueprint for future medicine, offering hope for millions affected by currently intractable diseases.
FAQs: Understanding the Medicine Nobel Prize 2025
Q: Who won the Nobel Prize in Physiology or Medicine 2025?
A: The 2025 medicine nobel prize 2025 was jointly awarded to three scientists: Mary E. Brunkow (USA), Fred Ramsdell (USA), and Shimon Sakaguchi (Japan).
Q: What was the specific discovery recognized by the Nobel Assembly?
A: The laureates were recognized for their “discoveries concerning peripheral immune tolerance,” specifically identifying Regulatory T-cells (Tregs) and the Foxp3 gene that controls them, which together prevent the immune system from attacking the body’s own tissues.
Q: Why are Regulatory T-cells called the immune system’s “security guards”?
A: They function as peacekeepers that actively patrol the body, monitoring other immune cells. They suppress any T-cells that become self-reactive, thereby maintaining the critical balance between defense against pathogens and tolerance for the body’s own healthy cells.
Q: What major diseases could benefit from this research?
A: This research has direct implications for developing better treatments for all autoimmune diseases (like Type 1 diabetes and rheumatoid arthritis), improving the success rate of organ transplants, and creating more effective cancer immunotherapies by targeting the Tregs that protect tumors. For official Nobel updates, please check the Karolinska Institutet website.
Conclusion
The work of Mary E. Brunkow, Fred Ramsdell, and Shimon Sakaguchi has provided a definitive answer to one of immunology’s most enduring mysteries: how the body stops its own internal defense system from turning against itself. By identifying Regulatory T-cells and the vital Foxp3 gene, they have paved the way for a new generation of sophisticated medical treatments. The transition from basic biological insight to tangible clinical applications, particularly in fighting autoimmune disorders and enhancing cancer immunotherapy, underscores the profound global impact of the medicine nobel prize 2025.
The journey from Shimon Sakaguchi’s initial, controversial observation to global recognition highlights the patience and tenacity required for fundamental scientific breakthroughs. Moving forward, will future therapies focus more on boosting these Tregs to stop autoimmunity, or on inhibiting them to cure cancer?
Source Article Credit: Information synthesized from the Nobel Assembly at Karolinska Institutet, The Hindu, and NDTV coverage of the 2025 Nobel Prize in Physiology or Medicine.